Modafinil is widely prescribed for sleep–wake disorders and is increasingly discussed among people exploring cognitive enhancers. For most patients, it is well tolerated. However, a small subset of users experience skin reactions, ranging from mild rashes to rare but serious conditions such as Stevens–Johnson syndrome (SJS).
As the official medical content voice of UkModafinil, our goal is to explain these risks clearly, compassionately, and with scientific accuracy—so readers can make informed, safety-first decisions.
Understanding Modafinil and Its Clinical Use
Modafinil belongs to a class of wakefulness-promoting agents known as eugeroics. It is approved for conditions such as narcolepsy, obstructive sleep apnoea–related excessive daytime sleepiness, and shift work sleep disorder. In the UK context, availability and regulation differ from country to country, which is why many readers search for information on Modafinil in the UK specifically.
In clinical practice, physicians often observe that modafinil’s mechanism—modulating dopamine transporters and influencing histamine, orexin, and glutamate pathways—offers alertness without the classical stimulant “crash.” Yet, like all centrally acting medicines, it carries potential adverse effects.
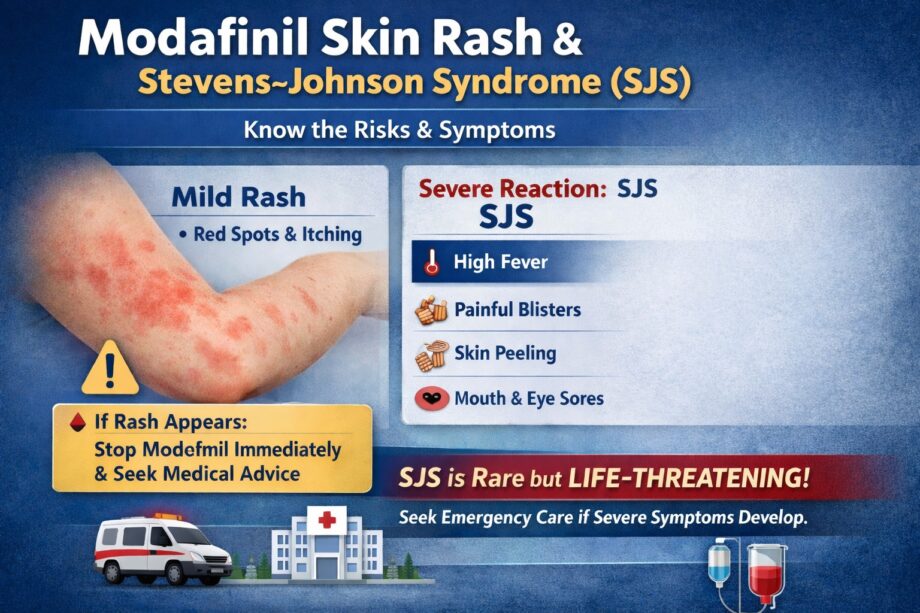
What Is a Modafinil-Related Skin Rash?
Mild to Moderate Reactions
Most modafinil-associated skin rashes are mild. These may appear within the first 1–3 weeks of treatment and commonly include:
- Red or pink patches on the trunk or limbs
- Itching or mild burning sensations
- Dry or slightly scaly skin
Such rashes are usually self-limiting once the drug is discontinued. In outpatient settings, clinicians often advise stopping modafinil immediately and monitoring symptoms rather than attempting to “push through” a rash.
Why Early Recognition Matters
Even seemingly mild rashes deserve attention. Regulatory agencies consistently warn that skin reactions can precede more serious immune-mediated conditions. The challenge is that early symptoms of dangerous reactions may look deceptively benign.
Stevens–Johnson Syndrome (SJS): A Rare but Serious Risk
What Is SJS?
Stevens–Johnson syndrome is a severe, potentially life-threatening hypersensitivity reaction. It involves widespread skin and mucous membrane damage, often triggered by medications.
According to the U.S. Food and Drug Administration (FDA), modafinil has been associated with rare cases of SJS, toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS). These warnings are clearly stated in the FDA-approved label for modafinil, which you can review directly here:
https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020717s032lbl.pdf
Key Symptoms to Watch For
SJS typically develops within the first few weeks of starting modafinil. Warning signs include:
- Fever or flu-like symptoms before a rash appears
- Painful red or purplish skin that spreads rapidly
- Blistering of the skin or mucous membranes (mouth, eyes, genitals)
- Skin peeling or shedding
In hospital dermatology units, physicians often note that patients describe pain rather than itch—an important red flag distinguishing SJS from simple allergic rashes.
How Common Is Stevens–Johnson syndrome With Modafinil?
SJS is extremely rare, but its severity warrants strict precautions. Post-marketing surveillance data suggest that serious skin reactions occur in far fewer than 1 in 10,000 users. Nonetheless, because outcomes can be severe, regulators emphasise zero tolerance for continuing treatment after any unexplained rash.
The European Medicines Agency (EMA) reinforces this stance, advising immediate discontinuation of modafinil at the first sign of rash unless the rash is clearly not drug-related:
https://www.ema.europa.eu/en/documents/product-information/modafinil-epar-product-information_en.pdf
Are Certain Modafinil Brands Riskier Than Others?
Patients sometimes ask whether generic or branded versions differ in safety. From a pharmacological standpoint, all approved formulations contain the same active compound. This includes commonly discussed options listed under Modafinil brands.
In clinical observation, the risk of SJS appears linked to individual immune response rather than the manufacturer. However, product quality, excipients, and regulatory oversight still matter—especially when sourcing medications online.
Who Is at Higher Risk of Severe Skin Reactions?
While SJS can occur unpredictably, some factors may increase risk:
- History of drug-induced rashes or SJS
- Certain genetic predispositions affecting immune response
- Concurrent use of other high-risk medications (e.g., specific anticonvulsants or antibiotics)
Importantly, children and adolescents appear to have a higher reported incidence of serious skin reactions with modafinil, which is why paediatric use is generally restricted or avoided.
What to Do If a Rash Appears
Immediate Steps
If you develop any rash while taking modafinil:
- Stop the medication immediately (unless advised otherwise by a clinician).
- Seek medical evaluation—especially if fever, eye pain, or mouth sores are present.
- Do not restart modafinil unless a healthcare professional explicitly confirms it is safe.
In emergency medicine, early withdrawal of the offending drug is one of the strongest predictors of better outcomes in SJS.
Do Not Self-Treat
Using antihistamines or topical steroids without medical advice can delay diagnosis. In suspected SJS, rapid referral to hospital care—often a burns or intensive dermatology unit—is standard practice.
Regulatory and Safety Guidance at a Glance
| Authority | Key Guidance |
|---|---|
| FDA | Discontinue modafinil at first sign of rash; risk of SJS/TEN documented |
| EMA | Similar warning; avoid re-challenge after rash |
| NHS (UK) | Advise urgent medical care for blistering or mucosal involvement |
The National Institutes of Health (NIH) also discuss severe cutaneous adverse reactions in their drug safety overviews, providing context on immune-mediated drug responses:
https://www.ncbi.nlm.nih.gov/books/NBK459323/
Balancing Benefits and Risks: A Practical Perspective
From a real-world clinical viewpoint, most patients who discontinue modafinil due to a rash recover fully with no long-term effects. The key is vigilance. When prescribed responsibly and monitored appropriately, modafinil remains a valuable therapeutic option for many.
At UkModafinil, we emphasise that wakefulness agents—and smart drugs more broadly—should never be approached casually. Understanding rare risks like SJS is part of using any medication responsibly.
Transparent Safety Disclaimer
This article is for educational purposes only and does not replace personalised medical advice. Always consult a qualified healthcare professional before starting, stopping, or resuming modafinil or any prescription medication.
Key Takeaway
- Mild skin rashes can occur with modafinil and often resolve after stopping the drug.
- Stevens–Johnson syndrome is very rare but serious.
- Any unexplained rash warrants immediate discontinuation and medical review.
- Early action saves lives.